Cupric oxide supplement
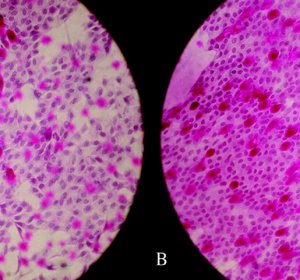
John R. Dunbar, UC Cooperative Extension Livestock Nutritionist James G. Morris, Veterinary Medicine and Physiological Sciences Ben B. Norman, UC Davis A. J. Jenkins, Colorado State University Charles B. Wilson, Sutter-Yuba counties John M. Connor, UC Sierra Foothill Research Extension Center publication information California Agriculture 47(3):25-26. . author affiliations…
☕ Read moreBlack Oxide services
We have done business with them for a number of years and when the daughter took it over, the customer service went downhill almost immediately. We sent our driver in to drop off our parts and asked them to hold off for an hour on processing our order because we had more parts to add. When I picked up the completed order (9 days later), they handed me the bill and we were charged…
☕ Read moreAluminum oxide Lewis structure
Lewis Structures of Atoms The chemical symbol for the atom is surrounded by a number of dots corresponding to the number of valence electrons. Lewis Structures for Ions of Elements The chemical symbol for the element is surrounded by the number of valence electrons present in the ion . The whole structure is then placed within square brackets, with a superscript to indicate…
☕ Read moreCopper I oxide chemical formula
The procedure that can be followed when confronted with the name of a compound and you wish to write its formula is as follows: 3. Balance the total positive and negative charge on the cation and anion. You ask yourself do the total positive charge and total negative charge add up to zero. If the answer is no then we ask how many of each ion must we have in order to balance…
☕ Read moreTitanium dioxide Coating
Chemours has been a pioneer in titanium dioxide technology for the coatings industry and ranks 1st among titanium dioxide manufacturers in product quality, customer service, and production capacity. The goal of our business is to utilize the breadth of capabilities and depth of knowledge intrinsic to Chemours to enable our customers to be more successful. By doing so Chemours…
☕ Read moreMagnesium oxide laxative
Alternative health practitioners know the benefits of period internal cleansing to maintain overall health and wellness. Magesium oxide is a safe and extremely effective substance to help achieve this. When the compound mixes with water, it essentially frees “blocked” oxygen in the intestinal tract. The additional oxygen promotes aerobic bacteria to grow (“friendly flora”)…
☕ Read moreWhere to Find zinc oxide powder?
So you may be wondering why I use a synthetic substance, zinc oxide, in my products if I’m trying to be all natural. You may not even know exactly what zinc oxide is. I definitely didn’t until doing some extensive research as I started creating baby products. As soon as I started down this crunchy path creating my own beauty and household products, I decided that I wanted to…
☕ Read moreBlack Oxide Kits
Being certifiably out of my mind, when I endeavor to do strange and unusual things in my shop, it rarely raises any eyebrows. However, my latest little foray into the unknown has a few of my car-guy buddies rather enthused. Specifically, I spent several months complaining about the apparent difficulty and cost associated with getting parts coated in black oxide for corrosion…
☕ Read moreBlack Oxide Industries
Black Oxide Coating process was originally developed during the early 1900’s. The modern bath become commercially prevalent during the later 1930’s and has remained so through today. With little advancement to the actual chemical process, Black Oxide Industries commitment to continuous improvement in techniques, education, and latest technology has advanced BOI to the top…
☕ Read moreOxidative Phosphorylation animation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate (ATP). Although the many forms of life on Earth utilize a range of different nutrients, almost all carry out oxidative phosphorylation to produce ATP, the molecule that supplies energy to metabolism. This pathway is probably so pervasive…
☕ Read moreAmmonia oxidizing archaea
In their natural habitats, microorganisms are often exposed to periods of starvation if their substrates for energy generation or other nutrients are limiting. Many microorganisms have developed strategies to adapt to fluctuating nutrients and long-term starvation. In the environment, ammonia oxidizers have to compete with many different organisms for ammonium and are often…
☕ Read moreCopper oxide sulfuric acid
The two main ingredients are copper and sulphuric acid, but simply adding copper to a diluted acidic solution will not promote the oxidation reaction. This is because the standard reduction potential of copper (+0.52) is higher that hydrogen (0.00), which means copper has a lower tendency to lose electrons than hydrogen. In practical terms, this means copper cannot reduce hydrogen…
☕ Read moreMagnesium oxidation number
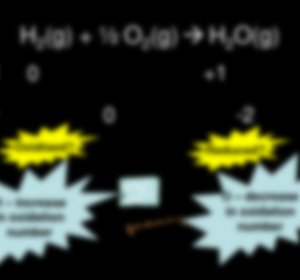
Part 1 of 2: Assigning Oxidation Numbers Based on Chemical Rules Determine whether the substance in question is elemental. Free, uncombined elemental atoms always have an oxidation number of 0. This is true both for atoms whose elemental form is composed of a lone atom, as well as atoms whose elemental form is diatomic or polyatomic. For example, Al(s) and Cl2 both have oxidation…
☕ Read more80 Grit aluminum oxide
Woodstock D1263 6-Inch by 80-Inch 60 Grit Aluminum Oxide Sanding Belt, 2-Pack 60 Grit 6-Inch by 80-Inch 2 Pack Aluminum oxide sanding belts For This Month. We gives you a good solid variety associated with Woodstock D1263 6-Inch by 80-Inch 60 Grit Aluminum Oxide Sanding Belt, 2-Pack with rapidly and Free postage on qualified items. This online shop are generally in associated…
☕ Read moreDecabromodiphenyl oxide
CAS 1163-19-5 DECABROMODIPHENYL ETHER msds toxicity property. This MSDS was developed for the Rip Stop Silver Jacket Flexduct. Also offer free database of 1163-19-5 including MSDS sheet(poisoning, toxicity, hazards and. Decabromodiphenyl Oxide, Brominated Flame Retardant, 82% Bromine. Decabromodiphenyl oxide (1163-19-5) SARA 313: 1.0 % de minimis concentration. As a supplier…
☕ Read moreNitrous oxide adverse effects
You might have heard a lot about the epidural as a form of pain relief… but you may not have heard as much about the return of a different kind of pain relief -nitrous oxide, commonly known as laughing gas. Commonly used in the UK and throughout the rest of Europe, nitrous oxide is self administered and is made of a 50/50 blend of nitrous oxide and oxygen. Surprised it wasn’t…
☕ Read moreOxidation number of calcium
20 February 2009 Chemists in Germany and Switzerland have discovered the first stable complex of calcium(I) - a highly unusual structure for a metal whose chemistry is normally dominated by the +2 oxidation state. Matthias Westerhausen and colleagues at the Friedrich Schiller University in Jena have found that two calcium(I) ions can be stabilised by inserting an arene group…
☕ Read moreCreatine VS nitric oxide
Shortly after our return from Detroit, we began 8 months of “medical HELL”. During this time, my love for her intensified so much I wanted to explode. At 4:16 AM on my bride left my arms for Heaven. The treatment of dandruff conditions is another common use for sulfur 8. As a lotion shampoo treatment, muscle advance creatine vs nitric oxide , sulfur 8 helps restore normal hair…
☕ Read moreWhat foods contain nitric oxide?
Nitric oxide is essential for the body to work correctly. Increasing the amount of nitric oxide your body produces can easily be done by eating the right foods with the right nutrients and vitamins. When doing research it’s important to also look nitric oxide up by its scientific name, arginine-alpha-keto-glutarate (AAKG). While eating foods to increase nitric oxide is beneficial…
☕ Read moreCalcium oxide
Synonyms: Burned lime; Burnt lime; Lime, Pebble lime; Quicklime; Unslaked lime OSHA IMIS Code Number: 0520 Chemical Abstracts Service (CAS) Registry Number: 1305-78-8 NIOSH Registry of Toxic Effects of Chemical Substances (RTECS) Identification Number: EW31 Department of Transportation Regulation Number (49 CFR 172.101) and Guide: 1910 157 chemical description, physical properties…
☕ Read moreCopyright © · All Rights Reserved | RSS | XML Sitemap