Mass of Magnesium oxide
Magnesium is weighed and then heated in a crucible. It reacts with oxygen to produce the oxide. It can be shown that there has been an increase in mass. The results can be used to find the formula of magnesium oxide and two methods are described for doing so.
Lesson organisation
The practical activity takes around 30-45 minutes, depending on the competence of the class. Students should all be standing and should wear eye protection. Students with long hair should tie it back.
It is a good idea for students to practice lifting the lid on and off the crucible and the crucible off the pipe clay triangle before they start. This has the added bonus of checking that all the tongs are functioning correctly.
To enable students to light their Bunsen burners they will need access to matches or lighters. Alternatively, light one or two Bunsen’s around the room and students can light their own using a splint.
The most significant hazard in this experiment is the hot apparatus. Warn students that it will take some time to cool down.
For classes with shorter attention spans, the final step of heating to constant mass could be omitted.
Chemicals
Magnesium ribbon, about 10-15 cm
Refer to Health & Safety and Technical notes section below for additional information.
Apparatus
Eye protection
Per pair or group of students:
Crucible with lid
Tongs
Pipe clay triangle
Bunsen burner
Tripod
Heat resistant mat
Emery paper (optional)
Access to:
Balance (2 d.p.)
Health & Safety and Technical notes
Wear eye protection.
Magnesium ribbon, Mg(s) - see CLEAPSS Hazcard. Fresh, clean magnesium is best for this experiment. If the magnesium is tarnished then emery or sand paper will be required to clean it.
Procedure
a Cut a piece of magnesium about 10-15 cm long. If it is looking tarnished or black then clean it using the emery paper. Twist it into a loose coil.
b Weigh the crucible with the lid (mass 1) and then the magnesium inside the crucible with the lid (mass 2).
c Set up the Bunsen burner on the heat resistant mat with the tripod. Place the pipe clay triangle over the tripod in a ‘star of David’ formation, ensuring that it is secure. Place the crucible containing the magnesium in the pipe clay triangle and put the lid on.
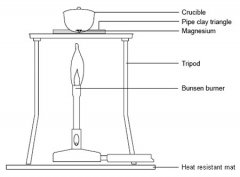
d Light the Bunsen burner and begin to heat the crucible. It is best to start with a gentle blue flame, but you will need to use a roaring flame (with the air hole fully open) to get the reaction to go.
Once the crucible is hot, gently lift the lid with the tongs a little to allow some oxygen to get in. You may see the magnesium begin to flare up. If the lid is off for too long then the magnesium oxide product will begin to escape. Don't let this happen.
You might also like


|
Anion self diffusion in magnesium oxide determined through ion-probe mass spectrometry (Massachusetts Institute of Technology. Dept. of Materials Science and Engineering. Thesis. 1977. M.S) Book |
|
Naturo Nitro Pre Workout Octane - Maximize Your Training with Massive Muscle Building Power for Any Fitness Level! Ignites a Body Building Construction Project with Every Workout - A Precision Formulated, Preworkout Performance Blend of Select Amino Acids Teams with a Vein-bulging, Triple-action Creatine Blend to Drive Your Muscle Gain and Workout Results to the Extreme - With Naturo Nitro Octane, Your Pre-workout Is Super Charged with a Proprietary, Jungle Crazed Energy and Focus Blend Combining Eight of Nature's Premier, Energy Accelerating Compounds, 28 Servings Pink Lemonade Health and Beauty (Naturo Sciences)
|

|
Enzymatic Therapy - Osteoprime Plus, 120 Tablets Health and Beauty (Enzymatic Therapy)
|

|
Doctor's Best Multiple Nutritional Supplement, 90 Count Health and Beauty (Doctor's Best)
|
|
Enzyte MRC | Testosterone Support, Muscle + Strength, Energy Booster, Increased Workout Capacity, NO2 Booster - Fenugreek, Rhodiola, Vitamin D3, NAC - 1 Month Supply (60 Capsules) Health and Beauty (Vianda)
|





