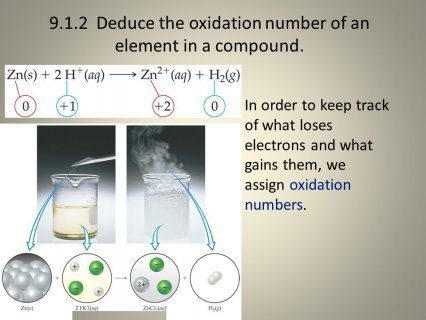
What is the definition of oxidation number?
The oxidation number reflects the (usually integral) unit measure of charge an atom in a molecule can expect to observe in a given chemical environment. For example, Hydrogen, is with near universality identical in its tendency to assume a +1 oxidation state. This "+1" is may be interpreted as a reflection of its electron-sharing behavior in molecular matter. Recall:
- First, molecular matter, as distinguished from ionic or metallic matter for example, characteristically possesses the trait of sharing of charge. This does not have to be equal sharing by any means, however, and that statement itself lends itself to a discussion of electronegativity (i.e., an element's inherent strength of polarization of negative charge toward itself). According to the Pauling scale, luorine, is the most electronegative element; it is interesting to note that its electronegativity value of 4.0 was arbitrarily assigned to it by Linus Pauling, and all the other elements' values calculated comparatively. In other words, the electronegativity scale is a relative measure, not a table of absolute, nominal data. (The table below has just a slight skew; it should be downshifted 0.1 units).
- Positive oxidation states, just like measures of charge themselves, are understood as possessing less charge despite our familiarity with magnitudes increasing along the positive axis in maths for example. This is because electric charge, carried by the electron (which is a lepton, or a fundamental particle in the Standard Model), is inherently negative. Therefore, positive values indicate the absence of electric charge.
Similar to hydrogen, oxygen is also observed to possess an identical value in almost all situations. It should not be surprising to hear that this oxidation number is -2 (since the addition of 2 electrons to a neutral oxygen atom brings completes its octet in the valence electron shell indicated by the principal quantum number, n = 2. There are, however, notable exceptions. One of the most widely known is hydrogen peroxide, H2O2. Here, oxygen possesses a -1 oxidation number. Notice that the oxidation numbers of hydrogen and oxygen annihilate:
2 x -> 2 x (+) = +2
2 x -> 2 x (-1) = -2
__________________
(+2) + (-2) = 0
Lastly before continuing, compare the structure of water (H2O) to that of the aforementioned anomaly, hydrogen peroxide (below, right). You will immediately see that water is completely in line with the expected formal oxidation numbers of both oxygen and hydrogen.
This should be the case. In fact, one will observe that for formal charges, the same is true. Namely, the sum of each of the atoms' formal charges in a molecule must yield the net charge on the molecule. By their interpretations, the oxidation numbers and the formal charges have a similar likening: they both tend to reflect atoms' individual propensity toward the acceptance/polarization or donation/sharing of charge density. Importantly, however, oxidation numbers should usually be reserved to considerations of acid-base systems or, more correctly, oxidation-reduction (a.k.a., "redox") reactions; I said acid-base just because nine times out of ten this is the chemical environment in which molecules undergo redox reactions.
You might also like




|
Rare earth uranium oxide, high grade unrefined uranium ore uraninite, for Geiger counter & radiation detector test source! BISS (LifeTech)
|

|
The rare earth uranium oxide, naturally radioactive euxenite-(Y) in granitic pegmatite, for Geiger counter & radiation detector test source! BISS (LifeTech)
|





