Oxidation of alcohols
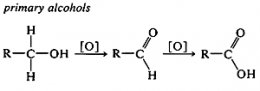 This presents various oxidation methods of alcohols. According to the scale of oxidation levels established for carbon (see Table 11-1), primary alcohols (RCH2OH) are at a lower oxidation level than either aldehydes (RCHO) or carboxylic acids (RCO2H). With suitable oxidizing agents, primary alcohols in fact can be oxidized first to aldehydes and then to carboxylic acids.
This presents various oxidation methods of alcohols. According to the scale of oxidation levels established for carbon (see Table 11-1), primary alcohols (RCH2OH) are at a lower oxidation level than either aldehydes (RCHO) or carboxylic acids (RCO2H). With suitable oxidizing agents, primary alcohols in fact can be oxidized first to aldehydes and then to carboxylic acids.
Unlike the reactions discussed previously in this chapter, oxidation of alcohols involves the alkyl portion of the molecule, or more specifically, the C-H bonds of the hydroxyl-bearing carbon (the a carbon). Secondary alcohols, which have only one such C-H bond, are oxidized to ketones, whereas tertiary alcohols, which have no C-H bonds to the hydroxylic carbon, are oxidized only with accompanying degradation into smaller fragments by cleavage of carbon-carbon bonds.
Conversion of ethanol to ethanal is carried out on a commercial scale by passing gaseous ethanol over a copper catalyst at 300°:
At room temperature this reaction is endothermic with an equilibrium constant of about 10~22. At 300° conversions of 20%-50% per pass can be realized and, by recycling the unreacted alcohol, the yield can be greater than 90%.
 Another commercial process uses a silver catalyst and oxygen to combine with the hydrogen, which makes the net reaction substantially exothermic:
Another commercial process uses a silver catalyst and oxygen to combine with the hydrogen, which makes the net reaction substantially exothermic:
In effect, this is a partial combustion reaction and requires very careful control to prevent overoxidation. In fact, by modifying the reaction conditions (alcohol-to-oxygen ratio, temperature, pressure, and reaction time), the oxidation proceeds smoothly to ethanoic acid:
Reactions of this type are particularly suitable as industrial processes because they generally can be run in continuous-flow reactors, and can utilize a cheap oxidizing agent, usually supplied directly as air.
You might also like





|
20% Pure Vitamin C + E Hyaluronic Acid Serum for Face Rejuvenation (1 fl oz), Skin Tightening, Dark Spot Removal & Anti Wrinkle Anti Blemish. BEST Antioxidant Anti Aging Vitamins Stimulate Collagen for Skin Renewal. All Natural & Organic, Cruelty Free - Dr Trusted. Kleem Organics FREE Beauty eBook. 100% Risk Free Guarantee Beauty (Kleem Organics)
|






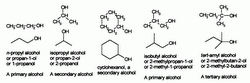 In chemistry, an alcohol is an organic compound in which the hydroxyl functional group (-OH) is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms.
In chemistry, an alcohol is an organic compound in which the hydroxyl functional group (-OH) is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms.