
Ascorbic acid oxidation
Canadian Journal of Chemistry, 1977, 55(15): 2799-2806, 10.1139/v77-389
Abstract
A polarographic study of the oxidation mechanism of L-ascorbic acid and of the reduction mechanism of dehydro-L-ascorbic acid was carried out in an acid medium.For L-ascorbic acid, the oxidation process involves a two electron transfer and obeys the overall reactionThe polarographic curve shows that the limiting current is governed by diffusion. On the rising portion of the wave, the two electron oxidation process consists of two consecutive one electron transfers, the second being the rate determining step (rds). The reaction orders, together with the Tafel slopes, were calculated.The reduction of dehydro-L-ascorbic acid at the limiting current is kinetically controlled and involves a two electron transfer. The reaction kinetic pathways were studied and the reaction orders and Tafel slope were calculated. It is deduced that, for low overvoltages, the second one electron transfer is the rate determining step.
|
Topical high-potency L-ascorbic acid: high-potency vitamin C products should be maintained at optimum conditions to minimize the oxidation rate and ... from: Household & Personal Products Industry Book (Thomson Gale) |
|
Differential selectivity in electrochemical oxidation of ascorbic acid and hydrogen peroxide at the surface of functionalized ormosil-modified electrodes [An article from: Analytica Chimica Acta] Book (Elsevier) |
|
|
Direct electron transfer of ferritin in dihexadecylphosphate on an Au film electrode and its catalytic oxidation toward ascorbic acid [An article from: Analytica Chimica Acta] Book (Elsevier) |






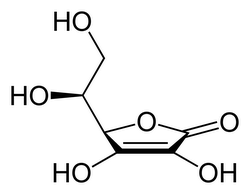 Vitamin C or L-ascorbic acid or L-ascorbate is an essential nutrient for humans and certain other animal species. In living organisms ascorbate acts as an antioxidant by protecting the body against oxidative stress. It is also a cofactor in at least eight enzymatic reactions including several collagen synthesis reactions that, when...
Vitamin C or L-ascorbic acid or L-ascorbate is an essential nutrient for humans and certain other animal species. In living organisms ascorbate acts as an antioxidant by protecting the body against oxidative stress. It is also a cofactor in at least eight enzymatic reactions including several collagen synthesis reactions that, when...