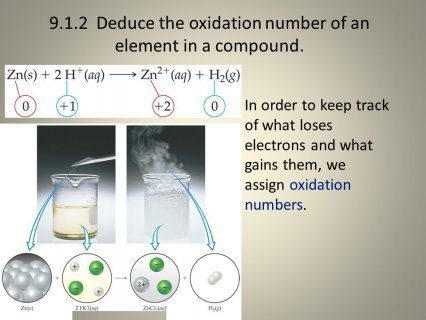
Oxidation number meaning
This segment lets go ahead and discuss the oxidation number method, so this will be really important when you're first learning how to do oxidation reduction reactions and as you go on through Chemistry so that you understand how the charges on different species kind of work. So oxidation number method is also referred to as oxidation states so you'll hear that come up repeatedly probably in your adventures with this topic. So what is the oxidation number method? Well it helps us keep track of the electrons that are gained when a substance is reduced and the electrons that are lost when the substance is oxidized. Remember that oxidation and reduction happen together they're oxidation reduction pairs if you will and I put OILRIG here as a reminder that oxidation is losing of electrons and reduction is gaining of electrons so this is always a handy tool to kind of write down and hope you keep track of what's happening.
So each atom in a neutral molecule or charged species is assigned what we call an oxidation number and that's the actual charge for a monatomic ion, remember a monatomic ion mono meaning just one so a singular ionic species. So the oxidation number of sodium atoms will change in a redox reaction alright that kind of make sense because there are electrons being transferred from one species to another so when oxidation occurs there's going to be an increase in the oxidation number. However, when reduction occurs there's going to be a decrease in the oxidation number again remember oxidation is losing of electrons so an increase in positive charge reduction is a gain of electrons so a decrease in positive charge.
So how do we actually assign oxidation numbers? So there are a few rules there's always exceptions to rules all of them that will have a chance to say here but these are the basics of what you need to note to get you started and increase your proficiency with this topic, so let's go ahead and see what they are. Understand that it will take you quite a bit of time before you become familiar and this feels like second nature for you so hopefully you won't get lost and bogged down in the details and just kind of be open to learning how to do it.
So the first one, is for an atom that's in it's elemental form, so basically the way you find it on the periodic table, it's going to have an oxidation number of 0 so for instance if we're talking about the diatomic chlorine gas Cl2 each chlorine atom in chlorine gas has an oxidation number of 0.
So for the second rule, for a monatomic ion the oxidation number is going to equal to charge on the ion, so for instance group I, your alkaline metals, when they, they're oxidized and so they're going to loose an electron to get a plus 1 charge. Group II they're going to loose 2 electrons to have a plus 2 charge. Your halogens, group VII, they're going to gain an electron to have a minus 1 charge.
|
Aspen Naturals Cocoa Butter (1 Lb) - Pure, Raw, Food-grade & Unrefined - Best Quality - Use for Lip Balm, Lotion, Cream, Oil, Stick or Body Butter - Make Your Own Formula -Treats Scars, Stretch Marks, Dry Skin and More Beauty (Aspen Naturals®)
|

|
Romantic Time Deluxe "I Love You" 18k Rose Gold Chunky Cuban Gemstone Accented Identification Bracelet Jewelry (Romantic Time)
|

|
Castrol 06249 EDGE 5W-40 SPT Synthetic Motor Oil - 1 Quart Bottle, (Pack of 6) Automotive Parts and Accessories (Castrol)
|

|
Mobil 1 98KF98 0W-20 Advanced Fuel Economy Synthetic Motor Oil - 1 Quart Automotive Parts and Accessories (Mobil 1)
|

|
Romantic Time Turquoise Stone Decorated Teardrop Strawberry Surfaced Heart To Heart Love 18k Rose Gold Link Bracelet Jewelry (Romantic Time)
|





