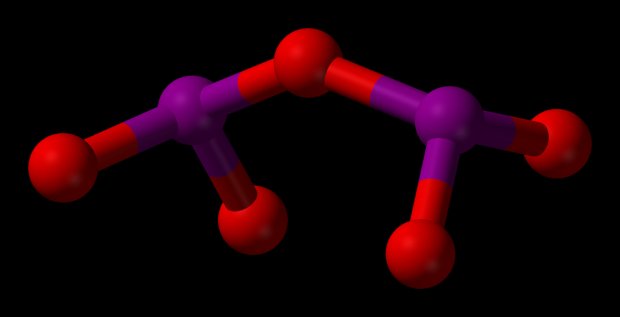
Chlorine oxide formula
Chlorine dioxide is a chemical compound with the formula CIO2, and is a popular disinfecting agent for treating both drinking water and water used in cooling systems. It was first introduced in 1944 by the Niagara Falls, New York Water Treatment Plant, and due to its superior use over chlorine when operating over a ph 7 with the presence of ammonia and amines, it quickly became widely used by 1956. Chlorine dioxide is also the most effective way to control biofilms in water distribution systems and to eliminate water pathogens such as the legionella bacteria. Below are just a few more ways that using chlorine dioxide will keep your water cooling system up and running.
Chlorine dioxide is an EPA registered biocide.
As long as it is used as directed, this biocide poses no unreasonable adverse effects on humans, the environment, or other non-target species.
It is not negatively impacted by pH.
Disinfectants can experience changes to their effectiveness depending on the pH of the water they are used in. Chlorine dioxide will not go through any fluctuations in strength, regardless of the pH.
Chlorine dioxide will not lose its effectiveness over time.
Meaning that bacteria will not grow resistant to it.
It is not negatively impacted by silica and phosphate.
This is vital since silica and phosphate are both commonly used as potable water corrosion inhibitors.
Chlorine dioxide is less corrosive than chlorine or other disinfection methods.
This keeps all equipment running smoothly, efficiently, and without incurring any additional costs to control corrosion.
You might also like




|
ATTITUDE Bathroom / Mold and Mildew Cleaner - 27.1 oz Home (Bio-Spectra)
|





