Reduced and Oxidized
The terms ionic and covalent describe the extremes of a continuum of bonding. There is some covalent character in even the most ionic compounds and vice versa.
It is useful to think about the compounds of the main group metals as if they contained positive and negative ions. The chemistry of magnesium oxide, for example, is easy to understand if we assume that MgO contains Mg2+ and O2- ions. But no compounds are 100% ionic. There is experimental evidence, for example, that the true charge on the magnesium and oxygen atoms in MgO is +1.5 and -1.5.
Oxidation states provide a compromise between a powerful model of oxidation-reduction reactions based on the assumption that these compounds contain ions and our knowledge that the true charge on the ions in these compounds is not as large as this model predicts. By definition, the oxidation state of an atom is the charge that atom would carry if the compound were purely ionic.
For the active metals in Groups IA and IIA, the difference between the oxidation state of the metal atom and the charge on this atom is small enough to be ignored. The main group metals in Groups IIIA and IVA, however, form compounds that have a significant amount of covalent character. It is misleading, for example, to assume that aluminum bromide contains Al3+ and Br- ions. It actually exists as Al2Br6 molecules.
This problem becomes even more severe when we turn to the chemistry of the transition metals. MnO, for example, is ionic enough to be considered a salt that contains Mn2+ and O2- ions. Mn2O7, on the other hand, is a covalent compound that boils at room temperature. It is therefore more useful to think about this compound as if it contained manganese in a +7 oxidation state, not Mn7+ ions.
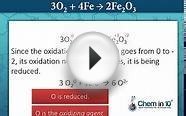
Let's consider the role that each element plays in the reaction in which a particular element gains or loses electrons..
When magnesium reacts with oxygen, the magnesium atoms donate electrons to O2 molecules and thereby reduce the oxygen. Magnesium therefore acts as a reducing agent in this reaction.
| 2 Mg | + O2 | 2 MgO |
| reducing
agent |
We can determine the relative strengths of a pair of metals as reducing agents by determining whether a reaction occurs when one of these metals is mixed with a salt of the other. Consider the relative strength of iron and aluminum, for example. Nothing happens when we mix powdered aluminum metal with iron(III) oxide. If we place this mixture in a crucible, however, and get the reaction started by applying a little heat, a vigorous reaction takes place to give aluminum oxide and molten iron metal.
You might also like



|
OION LB-8001 5-in-1 Air Cleaning System with True HEPA, UV-C, Ionizer, PCO Filtration, and Odor Reduction Air Purifier Home (OION)
|

|
Air Oasis AO3000 Surface and Air Sanitizer 3000 Sq Ft Coverage Home (EMG East, Inc. (direct order))
|

|
Vornado PCO300 Silverscreen Enhanced HEPA Air Purifier Home (Vornado Air LLC.)
|

|
Mammoth Air Purifier Q3 7 Stage Replacement Ice TechTM 4-in-1 Pre-Filter, HEPA, Carbon, & Photocatalytic Filter Home (Mammoth Air Purifier)
|

|
OION Photocatalytic Oxidation (PCO) Filter Replacement for LB-8001 Air Purifier Home Improvement (OION Technologies)
|






 Indium ( /ˈɪndiəm/ IN-dee-əm) is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows intermediate properties between these two. Indium was discovered in 1863 and named for the indigo blue line in its spectrum...
Indium ( /ˈɪndiəm/ IN-dee-əm) is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows intermediate properties between these two. Indium was discovered in 1863 and named for the indigo blue line in its spectrum...