Aluminum oxide melting point
 Aluminum Sulfate = Al(SO4)3 Sodium Hydroxide = NaOH
Aluminum Sulfate = Al(SO4)3 Sodium Hydroxide = NaOH
Word Equation : Aluminum Sulfate + Sodium Hydroxide yields Aluminum Hydroxide + Sodium Sulfate
Al2(SO4)3 + NaOH -> Al(OH)3 + Na2(SO4)
Balanced Chemical Equation : Al(SO4)3 + 6NaOH -> 2Al(OH)3 + 3Na2(SO4)
Visual Representation :Physical and Chemical Description Properties of Reactants : Aluminum Sulfate – Aluminum Sulfate can also be known as cake alum, fliter alum, and sulfuric acid. It has a melting point of 770 degrees celcius. It is slightly soluble in alcohol. Its has a pH level between 3.3 and 3.6, therefore it is acidic. Aluminum Sulfate is a solid, white crystalline and hygroscopic. Hygroscopy is the ability to hold water molecules together by absorbing the material. Sodium Hydroxide – Sodium Hydroxide is also known as caustic acid. It is very ionic and contains sodium cations and hydroxide anions. The hydroxide anions make it a strong base which causes it to react with acids to produce water and other salts. It is a white solid, mainly in the form of granules, pellets and flakes. It has a melting point of 318 degrees celcius.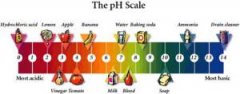 Its pH level is 13 therefore it is a very strong base.How Reactants are Obtained : Millosevichite is a rare mineral with the formula Al2(SO4)3 (Aluminum Sulfate). It can be found in volcanic environments and is known for burning coal dumps. If something is anhydrous is means it contains no water, Aluminum Sulfate is rarely found as an anhydrous salt. Sodium Hydroxide is industrially produced as a 50% solution by variations of the chloralkali process. The choloalkali process is the seperation of a Sodium Chloride solution. Chlorine gas is also produced in the process of choloalkali. Solid Sodium Hydroxide is obtained from this solution by the evaporation of water. In 2004, the worldwide production of Sodium Hydroxide was about 60 million tonnes.
Its pH level is 13 therefore it is a very strong base.How Reactants are Obtained : Millosevichite is a rare mineral with the formula Al2(SO4)3 (Aluminum Sulfate). It can be found in volcanic environments and is known for burning coal dumps. If something is anhydrous is means it contains no water, Aluminum Sulfate is rarely found as an anhydrous salt. Sodium Hydroxide is industrially produced as a 50% solution by variations of the chloralkali process. The choloalkali process is the seperation of a Sodium Chloride solution. Chlorine gas is also produced in the process of choloalkali. Solid Sodium Hydroxide is obtained from this solution by the evaporation of water. In 2004, the worldwide production of Sodium Hydroxide was about 60 million tonnes.
Conditions for Reaction to Occur : Both Aluminum Sulfate and Sodium Hydroxide are soluble. For the reaction we show them in the aqueous state. By mixing these two solutions together, it produces two new compounds, Aluminum Hydroxide and Sodium Sulfate. The Aluminum Hydroxide is considered insoluble and will therefore precipitate out as crystals. The Sodium Sulfate is soluble and will therefore remain dissolved and in solution.
You might also like











