Nitrous oxide Molecule
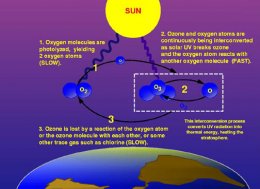
The ozone-oxygen cycle in the Earth’s stratosphere
[social_buttons]
The destruction of the Earth’s protective ozone layer (and the growth of the “hole” in this layer over the South Pole) due to the action of human-made chemicals was the leading environmental issue of the last century (entering the public lexicon sometime in the mid 1980’s), and no doubt prompted wider concerns about “greenhouse” effects and global warming that occupy so much climate science reporting today. The main (or most publicized) culprit of this ozone loss was a chemical called chlorofluorocarbon (CFC)*. But now, there’s a new leader in ozone destruction: nitrous oxide (N2O, also known as “laughing gas”), and its increasing concentration in the atmosphere is no laughing matter.
The Montreal Protocol (MP) on Substances That Deplete the Ozone Layer (adopted in 1987) was an outgrowth of the Vienna Convention for the Protection of the Ozone Layer (1985). The MP has been very successful at limiting the emissions of chlorine and bromine-containing halocarbons (halide elements combined with carbon) which have been, up until the end of the 20th Century, the dominant ozone-depleting substances (ODS).
But in a new NOAA study (Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century, Ravishankara/Daniel/Portman, reported in Science, 2 October, 2009) analyzing and calculating the ozone-depleting potential (ODP) of various GHGs, it was N2O that was found to be the new, leading culprit of ozone (O3) depletion in the upper stratosphere. The ODP is defined as the ratio of O3 destroyed by a unit mass of a chemical at the Earth’s surface to the amount of O3 destroyed by a unit mass of CFC (the ODS standard gauge).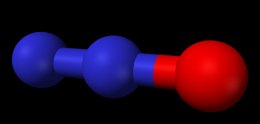 Their calculations allowed them to also determine the global warming potential (GWP) of each chemical emission.
Their calculations allowed them to also determine the global warming potential (GWP) of each chemical emission.
Model of a nitrous oxide molecule’s (N2O) dimensions – Blue spheres are nitrogen atoms, red sphere is oxygen.
Nitrogen oxides (NOx; any combination of nitrogen and one or more oxygen atoms), such as those created by the combustion of air and jet fuel, chemically react with ozone (O3) and then go through a short series of transformations, resulting in a single oxygen (O) molecule and one O3 molecule. But due to chemical affinities (oxygen in the air prefers its O2 state), this new O3 is short-lived, and quickly splits up and forms (2) O2 molecules.
The main source of these nitrogen oxides is nitrous oxide emitted at the surface (within the troposphere) and which is then transported to the upper stratosphere via air current circulation (this transport usually takes from 4-5 months).
You might also like

![PSX Longplay [027] N2O: Nitrous Oxide - Part 1 of 3](/img/video/psx_longplay_027_n2o_nitrous_oxide.jpg)







 Nitrogen ( /ˈnaɪtrɵdʒən/ NY-trə-jən) is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.09% by volume of Earth's atmosphere. The element nitrogen was discovered as a separable...
Nitrogen ( /ˈnaɪtrɵdʒən/ NY-trə-jən) is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.09% by volume of Earth's atmosphere. The element nitrogen was discovered as a separable...