
Empirical formula of copper oxide
Students heat copper(II) oxide in a glass tube while passing methane over it. The copper(II) oxide is reduced to copper. If the reactants and products are weighed carefully the formula of the copper oxide can be deduced. This could also be used simply as an example of reduction.
Lesson organisation
This experiment is likely to take up to an hour, perhaps more to analyse the results. Students who have not carried out this type of reaction before may find it helpful to have the techniques demonstrated first. It is not really suitable for a class practical for students under the age of 14, but could be a useful demonstration.
Each pair or group of students will need access to 2 gas taps.
The students will need access to matches or lighters to light their Bunsen burners. Alternatively, light a few around the room and students can light their own using a splint.
Chemicals
Copper(II) oxide (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), 2 spatulas
Refer to Health & Safety and Technical notes section below for additional information.
Apparatus
Eye protection
Per pair of group of students:
Reduction tube, (hard glass test-tube with small hole near closed end)
1-hole bung with glass tube to fit the reduction tube
Rubber tubing
Clamp stand, boss and clamp
Bunsen burner
Heat resistant mat
Spatula
Balance – must be accurate to at least 0.01g
Health & Safety and Technical notes
Wear eye protection.
Copper(II) oxide, CuO(s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) - see CLEAPSS Hazcard. For best results use analytical grade copper(II) oxide which has been dried by heating in an open dish at 300–400 °C for 10 min and then stored in a desiccator.
It is also worth referring to the CLEAPSS Laboratory Handbook Section 13.2.3 for further information about this experiment.
Procedure
a Weigh the test tube with the bung in (mass 1). Put 2 spatulas of copper(II) oxide into the tube and spread it out as much as possible.
b Weigh the tube again, with the copper oxide in it (mass 2).
c Assemble the apparatus as shown in the diagram, but do not place the Bunsen burner underneath yet. Clamp the test tube as near to the bung as possible.
d Turn on the gas tap attached to the test tube about half way to get a steady flow of gas. This will pass methane through the apparatus.
e Wait for at least 10 seconds, to allow all the air to be flushed out of the tube and then light the gas coming out of the hole at the end of the tube. If this experiment is a student activity, a teacher should supervise this step. Take care not to lean over the tube as you light the gas. Adjust the gas tap so that the flame is about 3 cm high.
You might also like




|
ATTITUDE Bathroom / Mold and Mildew Cleaner - 27.1 oz Home (Bio-Spectra)
|








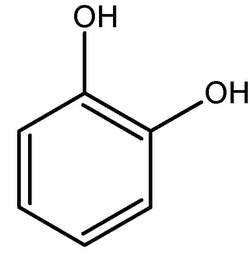 Catechol, also known as pyrocatechol or 1,2-dihydroxybenzene, is an organic compound with the molecular formula C6H4(OH)2. It is the ortho isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. About 20 million kg are produced annually, mainly as a precursor to pesticides, flavors, and...
Catechol, also known as pyrocatechol or 1,2-dihydroxybenzene, is an organic compound with the molecular formula C6H4(OH)2. It is the ortho isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. About 20 million kg are produced annually, mainly as a precursor to pesticides, flavors, and...