
Balanced equation for aluminum oxide
Chemistry is used in everyday life, from making food to breathing air. Chemical reactions are what creates life, makes food into energy, and even what causes food to rot. When… you balance equations, you are learning the amount of elements used in the reactants, or found in the products. If you do not balance the equation, you will only know what you started with, and not the final product.Chemistry is just like math when it comes to balancing equations. You want both sides of the equation to be the same. In Chemistry, this is done by making sure all atoms in your products can be found in the reactants.In a chemistry equation, the reactant is the material you start with. For example, when making water, your reactants would be two hydrogen atoms and one oxygen atom. The more complex the equation, the more reactants will be present.The product in a chemistry equation is the final product formed when the two reactants combine through a chemical reaction. Using the same example from above, when you combine two hydrogen atoms and one oxygen atom, the product would be water, or H20. It often takes a complex amount of reactants to create a product.When you are presented with an equation and must balance it, you will first want to identify all of the elements in the equation. This will give you a quick view of what will need to be added or subtracted from the equation to make it equal. The next step is to see what the charge is, or how many electrons are on each side. Then, start with one side of the equation and change the coefficients of the atom so that there will be the same number on both sides. Continue with this until you have all of the elements accounted for. Finally, check the charge on both sides of the equation.There are a few requirements to meet when properly balancing chemical equations. The first is making sure there is the same number of atoms on both sides of the equation. Atoms do not disappear or appear in a chemical reaction, so they must all be accounted for. By the same token, atoms in an element do not change to another element. Therefore, if you have hydrogen atoms on one side of the equation, you must have hydrogen atoms on the other side of the equation. Finally, the charge should be the same on both sides of the equation. This can be the hardest part of balancing equations. Unless it is a nuclear reaction, you cannot create nor destroy electrons in a reaction.While it seems to be a complicated process, balancing chemistry equations is fairly easy once you learn how. Knowing how reactants respond to various temperatures and pressures is how warning labels are made on common products, such as hairspray. Balancing equations ensures the reaction adheres to the conservation law of matter. That law states that elements cannot be created nor destroyed in an isolated system, or reaction.It is easiest to balance equations if you isolate the chemicals and then take an inventory of the elements. Many find this easiest if they draw a chart that shows the name of the element, the number before the equal sign, and then the number after the equal sign. The equation is not balanced until the numbers are the same. (MORE)
You might also like



|
Practical Tactical Pen - Always Be Prepared - a Discrete, High-Strength Aluminum Tactical Tool Combined with a Quality Pen with a Lightweight Ergonomic Design for Quick, Effective Use in Self Defense and Ease in Writing - Satisfaction Guaranteed Home Improvement (Pocket Partners)
|

|
6 Piece Jewelry Drill Set with Handle Art and Craft Supply (Jewelry Displays & Boxes)
|

|
JW Winco Aluminum 6063-T5 Clamping Knob, Steel Threaded Stud, 3/8"-16 Thread Size x 1-3/4" Thread Length, 2-1/2" Head Diameter (Pack of 1) BISS (JW Winco)
|

|
Chive 1600 Sports (Kershaw)
|

|
Artistic Wire Aluminum Craft Wire, 12 Gauge Thick, 12 Meter Spool, Natural Aluminum Home (Artistic Wire)
|






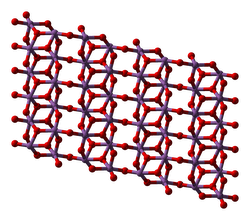 Antimony pentoxide (Sb2O5) is a chemical compound of antimony and oxygen. It always occurs in hydrated form, Sb2O5·nH2O. It contains antimony in the +5 oxidation state.
Antimony pentoxide (Sb2O5) is a chemical compound of antimony and oxygen. It always occurs in hydrated form, Sb2O5·nH2O. It contains antimony in the +5 oxidation state.