Antimony v oxide
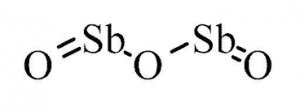 Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions only with hydrolysis.
Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions only with hydrolysis.
Antimony(III) oxide is an amphoteric oxide, it dissolves in aqueous sodium hydroxide solution to give the meta-antimonite NaSbO2, which can be isolated as the trihydrate. Antimony(III) oxide also dissolves in concentrated mineral acids to give the corresponding salts, which hydrolyzes upon dilution with water. With nitric acid, the trioxide is oxidized to antimony(V) oxide.
When heated with carbon, the oxide is reduced to antimony metal. With other reducing agents such as sodium borohydride or lithium aluminium hydride, the unstable and very toxic gas stibine is produced. When heated with potassium bitartrate, a complex salt potassium antimony tartrate, KSb(OH)2•C4H2O6 is formed.
Name:Antimony(III) oxide
EINECS:215-175-0
Molecular Formula:Sb2O3
Synonyms:Antimony trioxide; diantimony trioxide; Antimonyoxideelecgrwhitepowder; Antimonyoxidepowder
InChI:InChI=1/3O.2Sb/q3*-2;2*+3
Appearance:White Powder
Molecular Weight:291.52
Density:5.2
Boiling Point:1456°C
Melting Point:656°C (subl.)
Solubility:Slightly soluble
Stability:Stable.
Chemical Properties: White Powder
General Description: Diantimony trioxide is a white crystalline solid. Diantimony trioxide is insoluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Diantimony trioxide is used to fireproof fabrics, paper and plastics, as a paint pigment and for many other uses.
Air & Water Reactions: Insoluble in water.
Reactivity Profile: Idiantimony trioxide ignites and burns when heated in powdered form in air . Reacts violentlhy with bromine trifluoride.
Health Hazard :Dust: P oisonous if inhaled or if skin is exposed. If inhaled will cause coughing, difficult breathing or loss of consciousness. Sllid: Poisonous if swallowed or if skin is exposed. If swallowed will cause dizziness, nausea, vomiting or loss of consciousness.
Fire Hazard: Not flammable.
Uses:
The annual consumption of antimony(III) oxide in the United States and Europe is approximately 10, 000 and 25, 000 tonnes, respectively. The main application is for flame retardants in combination with halogenated materials. The combination of the halides and the antimony being key to the flame-retardant action for polymers, helping to form less flammable chars. Such flame retardants are found in electrical apparatus, textiles, leather, and coatings.
You might also like



|
Practical Tactical Pen - Always Be Prepared - a Discrete, High-Strength Aluminum Tactical Tool Combined with a Quality Pen with a Lightweight Ergonomic Design for Quick, Effective Use in Self Defense and Ease in Writing - Satisfaction Guaranteed Home Improvement (Pocket Partners)
|

|
6 Piece Jewelry Drill Set with Handle Art and Craft Supply (Jewelry Displays & Boxes)
|

|
JW Winco Aluminum 6063-T5 Clamping Knob, Steel Threaded Stud, 3/8"-16 Thread Size x 1-3/4" Thread Length, 2-1/2" Head Diameter (Pack of 1) BISS (JW Winco)
|

|
Chive 1600 Sports (Kershaw)
|

|
Artistic Wire Aluminum Craft Wire, 12 Gauge Thick, 12 Meter Spool, Natural Aluminum Home (Artistic Wire)
|






 Antimony pentoxide (Sb2O5) is a chemical compound of antimony and oxygen. It always occurs in hydrated form, Sb2O5·nH2O. It contains antimony in the +5 oxidation state.
Antimony pentoxide (Sb2O5) is a chemical compound of antimony and oxygen. It always occurs in hydrated form, Sb2O5·nH2O. It contains antimony in the +5 oxidation state.